Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Molecular Mechanism and Actions of Antifungal Drugs Using Trichosporon Fungal Species
Authors: Pooja Kumari, Sujata Kushwaha, C. K. Tyagi, Narendra Patel
DOI Link: https://doi.org/10.22214/ijraset.2024.64031
Certificate: View Certificate
Abstract
Antifungal agents play a crucial role in the treatment of fungal infections, which can range from minor skin conditions to life-threatening systemic diseases. These abstract reviews the pharmacological objectives, mechanisms of action, and clinical applications of antifungal agents. The primary objectives of antifungal therapy include achieving fungicidal or fungi static activity against fungal pathogens while minimizing toxicity to host cells. Despite the fact that Trichosporon spp. are probably the second or third most prevalent non-Candida yeast infection that causes invasive disease in hematological cancer patients. Central and vesical venous catheters, as well as devices related to peritoneal venous catheters, are the primary vectors of invasive Trichosporon spp. infection. Antifungal medications and host immune responses can be avoided by these organisms because they are adept at adhering to these devices and forming biofilms. Mechanisms of action vary widely among antifungal drugs, targeting fungal cell walls, membranes, nucleic acids, or metabolic pathways essential for fungal survival. Clinical efficacy is influenced by factors such as spectrum of activity, pharmacokinetic properties, and the emergence of resistance. Challenges in antifungal therapy include the rise of resistant fungal strains, limited treatment options, and the complexity of treating immune compromised patients. Future research directions include the development of novel antifungal agents, strategies to combat resistance, and improvements in diagnostic and therapeutic approaches. Overall, antifungal therapy continues to evolve, driven by ongoing research aimed at enhancing treatment outcomes and reducing the global burden of fungal infections.
Introduction
I. INTRODUCTION
Anti-fungal drugs target various molecular mechanisms to inhibit the growth or kill fungal cells. Here are some common molecular mechanisms targeted by anti-fungal drugs:
Cell Membrane Disruption: Some anti-fungal drugs, such as polyenes (e.g., amphotericin B) and azoles (e.g., fluconazole), disrupt fungal cell membranes. Polyenes tie to ergosterol, a critical part of parasitic cell films, framing pores that upset layer respectability and lead to cell passing. Azoles inhibit ergosterol synthesis by blocking the enzyme lanosterol demethylase, which is necessary for ergosterol production. Without ergosterol, fungal cell membranes become unstable and leaky, ultimately leading to cell death.
Cell Wall Synthesis Inhibition: Drugs like echinocandins (e.g., caspofungin) target the synthesis of β-(1,3)-D-glucan, a major component of the fungal cell wall. These drugs inhibit the enzyme
β-(1,3)-D-glucan synthase, which is responsible for synthesizing β-(1,3)-D-glucan. Without a functional cell wall, fungal cells become structurally weakened and prone to lysis.
Nucleic Acid Synthesis Inhibition: Some anti-fungal drugs interfere with nucleic acid synthesis in fungal cells. For example, 5-flucytosine (5-FC) is converted into 5-fluorouracil within fungal cells. 5-fluorouracil inhibits fungal RNA and DNA synthesis by interfering with nucleotide metabolism, ultimately leading to cell death.
Microtubule Disruption: Griseofulvin disrupts fungal cell division by inhibiting the function of microtubules. This disruption interferes with mitosis and leads to the inhibition of fungal growth.
Mitochondrial Function Inhibition: Drugs like azoles and amorolfine inhibit the function of cytochrome P450 enzymes, which are essential for the synthesis of ergosterol and other vital compounds in fungal mitochondria. These drugs hinder fungal metabolism and cause cell death by interfering with mitochondrial function.
Protein Synthesis Inhibition: Drugs such as sordarins inhibit fungal protein synthesis by targeting the elongation factor 2 (EF-2), which is involved in the ribosomal translocation step during protein synthesis. By inhibiting protein synthesis, these drugs disrupt essential cellular processes and lead to fungal cell death. Different classes of anti-fungal drugs may target one or more of these molecular mechanisms, and the choice of drug depends on factors such as the type of fungal infection, its severity, and the patient's medical history.
A. Types Of Fungal Infections
Fungal infections, which can spread to different parts of the body, are caused by a variety of fungi. Several common types of fungal infections include:
1) Cutaneous Fungal Infections: These infections affect the skin, hair, and nails. Examples include:
- Tinea corporis (ringworm): Ring-shaped rash on the skin.
- Athlete's foot (competitor's foot): Parasitic contamination of the feet, especially between the toes.
- Tinea cruris (jock itch): Fungal infection of the groin area.
2) Oral and Oesophageal Candidiasis: Caused by Candida species, these infections affect the mouth and throat. Commonly known as thrush, symptoms include white patches on the tongue and inner cheeks.
3) Vaginal Yeast Infection: Also caused by Candida species, these infections affect the vaginal area and may cause symptoms such as itching, burning, and abnormal discharge.
4) Systemic Fungal Infections: These infections can affect internal organs and may be life-threatening, especially in immune compromised individuals. Examples include:
- Invasive candidiasis: Candida bloodstream infection that can spread to various organs.
- Invasive aspergillosis: Caused by Aspergillus species, often affects the lungs and can spread to other organs.
- Cryptococcal meningitis: Infection of the membranes surrounding the brain and spinal cord, caused by Cryptococcus species.
5) Pneumocystis Pneumonia (PCP): Caused by Pneumocystis jirovecii, this fungal infection primarily affects individuals with weakened immune systems, such as those with HIV/AIDS.
6) Histoplasmosis: Caused by the fungus Histoplasma capsulatum, this infection is acquired by inhaling fungal spores found in soil containing bird or bat droppings. It can spread to different organs but primarily affects the lungs.
7) Blastomycosis: This infection, which is caused by Blastomyces dermatitidis, can also be contracted by inhaling soil-borne fungal spores, especially in wooded areas. It can spread to different organs yet generally influences the lungs.
8) Coccidioidomycosis (Valley Fever): Caused by Coccidioides species, this infection is endemic to certain areas with dry, arid climates. It can spread to different organs yet generally influences the lungs.
These are just a few examples of the many types of fungal infections that can occur. Treatment typically involves antifungal medications, which may be topical, oral, or intravenous.Depending on the severity and location of the infection.
II. CLASSIFICATION OF ANTIFUNGALS DRUGS
Antifungal drugs are agents used to treat fungal infections, which can affect various parts of the body including the skin, nails, respiratory tract, and systemic organs. These infections can be caused by different types of fungi, including yeasts, Molds, and dermatophytes. Classification of antifungal drugs are:
A. Polyenes
- Examples: Amphotericin B, Nystatin
- Mechanism of Action: binds to ergosterol in the membranes of fungal cells, causing pores to form, resulting in cell death.
- Uses: Treats severe systemic fungal infections (Amphotericin B) and topical infections (Nystatin).
B. Azoles
- Examples: Fluconazole, Itraconazole, Voriconazole, Ketoconazole
- Mechanism of Action: Inhibit the enzyme lanosterol 14-alpha-demethylase, blocking ergosterol synthesis, essential for fungal cell membrane integrity.
- Uses: Broad spectrum, treating various systemic and localized fungal infections.
C. Echinocandins
- Examples: Caspofungin, Micafungin, Anidulafungin
- Mechanism of Action: By inhibiting 1,3-D-glucan synthase, The synthesis of glucans by the fungal cell wall is hampered..
- Uses: Effective against Candida and Aspergillus species.
D. Allylamines
- Examples: Terbinafine, Naftifine
- Mechanism of Action: Reduce ergosterol synthesis by inhibiting squalene epoxidase.
- Uses: Primarily for dermatophyte infections (e.g., athlete’s foot, ringworm).
E. Pyrimidine Analogues
- Example: Flucytosine
- Mechanism of Action: Converted to 5-fluorouracil in fungal cells, interfering with RNA and DNA synthesis.
- Uses: Often used in combination with other antifungals for systemic infections.
F. Others
- Griseofulvin: Inhibits fungal mitosis by interacting with microtubules, used for dermatophyte infections.
- Tolnaftate: Used topically for dermatophyte infections
III. MOA OF ANTIFUNGAL DRUGS
- Polyenes (e.g., Amphotericin B, Nystatin)
- Mechanism: Polyenes bind to ergosterol, a key component of fungal cell membranes, forming pores that disrupt membrane integrity. Cell contents leak out, eventually leading to cell death.
- Spectrum: Broad spectrum against many fungal species.
- Azoles (e.g., Fluconazole, Itraconazole, Voriconazole)
- Mechanism: Azoles inhibit ergosterol synthesis by blocking the enzyme lanosterol demethylase, which is necessary for ergosterol production. Without ergosterol, parasitic cell films become shaky and defective, prompting cell demise.
- Spectrum: Varies depending on the specific azole, but generally broad spectrum against a wide range of fungal pathogens.
- Echinocandins (e.g., Caspofungin, Micafungin, Anidulafungin)
- Mechanism: By inhibiting the enzyme -(1,3)-D-glucan synthase, echinocandins prevent the synthesis of -(1,3)-D-glucan, a major component of the fungal cell wall.. This disrupts fungal cell wall integrity, leading to cell lysis.
- Spectrum: Active against Candida species and some other fungi, but not effective against Molds.
- Allylamines (e.g., Terbinafine)
- Mechanism: By blocking the enzyme squaleneepoxidase, which is involved in the initial steps of ergosterol biosynthesis, allylamines inhibit fungal ergosterol synthesis. This disrupts fungal cell membrane integrity and leads to cell death.
- Spectrum: Effective against dermatophyte fungi, such as those causing athlete's foot and nail infections.
- Flucytosine (5-FC)
- Mechanism: Flucytosine is converted into 5-fluorouracil within fungal cells. 5-fluorouracil interferes with fungal RNA and DNA synthesis by fragmenting nucleotide metabolism.
- Spectrum: Active against Candida species and Cryptococcus neoformans.
- Griseofulvin
- Mechanism: Griseofulvin disrupts fungal cell division by interfering with microtubule function, inhibiting mitosis and leading to inhibition of fungal growth.
- Spectrum: Effective against dermatophyte fungi, particularly those causing superficial infections of the skin, hair, and nails.
- Amorolfine
- Mechanism: Amorolfine inhibits the function of cytochrome P450 enzymes, disrupting ergosterol synthesis and interfering with mitochondrial function.
- Spectrum: Primarily used topically for dermatophyte infections.
- The major classes of antifungal medications and their respective mechanisms of action are listed below. Each drug class targets different aspects of fungal physiology, making them effective against various types of fungal infections.
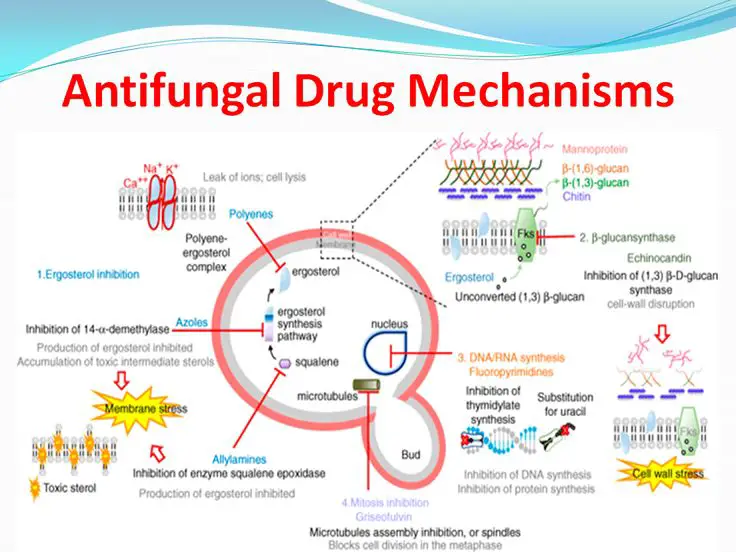
Figure 1: Antifungal drug mechanism
The current study used murine and Galleria mellonella as infection models to investigate Trichosporon infections. The developed animal models proved to be useful in the evaluation of virulence and antifungal efficacy for seven clinical isolates of Trichosporon asahii, T. asteroides, and T. inkin. The E-test and broth microdilution techniques were used in vitro to determine whether or not the Trichosporon isolates were susceptible to a number of common antifungal medications. Amphotericin had a lower minimal inhibitory concentration (MIC) in the E-test, while caspofungin had a slightly higher MIC. The MICs for the azoles were different but comparable between the two methods. In both the immunosuppressed murine models and the G. mellonella model, all three Trichosporon species caused infection. Both the murine and G. mellonella models showed species- and strain-dependent variations. In both animal hosts, it was demonstrated that T. asahii was more virulent than the other two species. In the murine model, virulence differences between T. asteroides strains were significant. When compared to the untreated control groups that were infected with any one of the three Trichosporon species, fluconazole and voriconazole were able to increase the survival rates of the animals in both animal models. Amphotericin did not reduce mortality in any of the three species of G. mellonella. Amphotericin, on the other hand, was able to decrease murine mortality in either the T. asahii or T. inkin models. As a result, the developed animal infection models can be directly applied to further research into the molecular factors that determine Trichosporon virulence and antifungal resistance.
IV. ADME OF ANTIFUNGAL DRUGS
- Pharmacokinetics: The body's reactions to the drug, such as absorption, distribution, metabolism, and excretion (ADME), are referred to as pharmacokinetics.
- Absorption: Antifungal drugs can be administered through various routes including oral, intravenous, topical, and sometimes intramuscular. Absorption rates vary depending on the route of administration and the specific drug.
- Distribution: Antifungal medications travel throughout the body via the bloodstream after being absorbed. Distribution depends on factors such as protein binding, tissue permeability, and drug lipophilicity.
- Metabolism: Many antifungal drugs undergo hepatic metabolism, primarily through the cytochrome P450 enzyme system. Drug toxicity and efficacy can be affected by metabolism.
- Excretion: Antifungal drugs and their metabolites are eliminated from the body mainly via renal excretion. The rate of excretion influences the duration of drug action and potential for drug accumulation.
- Pharmacodynamics: Pharmacodynamics refers to what the drug does to the body, including its mechanism of action, potency, and efficacy.
- Mechanism of Action: Different mechanisms are used by antifungal drugs to stop or kill fungi. Common mechanisms include interfering with fungal protein synthesis, compromising the integrity of the cell membrane, inhibiting fungal nucleic acid synthesis, or interfering with fungal cell wall synthesis.
- Potency and Efficacy: The potency of antifungal drugs refers to their ability to inhibit or kill fungi at a given concentrationThe drug's ability to produce the desired therapeutic effect in vivo is referred to as efficacy.
- Resistance: Fungal resistance to antifungal drugs is a significant concern. Resistance mechanisms can involve alterations in drug targets, decreased drug uptake, increased drug efflux, or enhanced drug metabolism. Monitoring for resistance and appropriate drug selection are crucial for successful treatment.
- Therapeutic Drug Monitoring (TDM): Some antifungal drugs, especially those with narrow therapeutic indices or significant interpatient variability, may require TDM. TDM involves measuring drug concentrations in the blood to optimize dosing and minimize toxicity.
Overall, understanding the pharmacokinetic and pharmacodynamic properties of antifungal drugs is essential for their safe and effective use in the treatment of fungal infections.
V. LITERATURE REVIEW
Filomena Nazzaro, Florinda Fratianni, Raffaele Coppola and Vincenzo De Feo in 2017 reviewed that;The fungicide and fungistatic properties of essential oils, as well as the growing body of literature on their mechanisms of action and knowledge of their traditional and new uses, highlight the potential applications of these natural substances in numerous fields, including human medicine, agriculture, food technology, and reducing the use of synthetic drugs and additives. The use of essential oils conforms to the search for natural substances that are safe for human and environmental health, despite the need for additional research. Focal areas deserving further detailed studies appearthe evaluation of the possible synergistic effects among the EOs and/or their components and among essential oils and synthetic molecules; the identification of the active components.
Zhenzhen Zhao, Qiushuo Wang, Kaimei Wang, Kemp Brian, Changhong Liu, Yucheng Gu has mentioned that B. vallismortis ZZ185, an endophytic bacterium, has been isolated from healthy Broadleaf Holly stems. Both the culture filtrate and the n-butanol extract demonstrated high efficiency in in vivo assays involving wheat seedlings infected with A. alternata and F. graminearum, as well as strong in vitro inhibition activity against plant pathogens such as F. graminearum, A. alternata, R. solani, C. parasitica, and P. capsici. Subsequent investigation revealed that the two relatively thermostable Bacillomycin D compounds were combined to produce the antifungal activity in the n-butanol extract of the culture filtrate of strain ZZ185.
Sortino M, Delgado P, Juárez S, Quiroga J, Abonía R, Insuasty B, Nogueras M, Rodero L, Garabito FM, Enriz RD, Zecchino SA worked onSynthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorganic & Medicinal Chemistry. 2007; the study reports on the successful synthesis of new (Z)-5-arylidenerhodanines using a microwave without the need for solvents.The in vitro antifungal activity of these compounds was assessed using the CLSI (previously NCCLS) guidelines against a panel of clinical opportunistic pathogenic fungi as well as standardized ones. The results of computational studies and an analysis of the structure-activity relationship (SAR) revealed that the most active compounds, F- and CF3-substituted rhodanines, had low polarizability and high logP values. Mechanism-based assays indicate that the active compounds would neither bind to ergosterol nor inflict membrane damage.
Yuki Kurita, Makoto Miyaji, Ryuichiro Kurane and Yoshimasa Takahara reported in their review; The antifungal activity of aromatic aldehydes and their calculated LEMO or HOMO energy level were difficult to correlate, in contrast to the aliphatic aldehydes. It's possible that aromatic aldehydes must also possess hydrophobicity and cell-membrane permeability in order to have a potent antifungal effect.
Emma Nelly Quiroga, Antonio Rodolfo Sampietro; Marta Amelia Vattuone demonstrated that Various auxiliary metabolites delivered by plants are turning out to be increasingly more huge because of their potential biotechnological utilizes. Ten Argentinean spices utilized in conventional medication have their antifungal adequacy in their ethanolic removes reported. Among the antifungal assays used were disk and well diffusion assays, growth inhibition by broth dilution tests, and radial growth inhibition. Basidiomycetes, yeasts, and fungi that are responsible for wood rot were chosen as the test fungi. Extracts of Larrea divaricate, Zuccagniapunctata, and Larreacuneifolia demonstrated impressive activity in tests against the majority of the test fungi. In addition to the aforementioned plants, Prosopancheamericana stopped yeast from growing.
Qingfei Liu, Walter Luyten, Klaartje Pellens, Yiming Wang e Wei Wang, Karin Thevissen, Qinglin Liang, Bruno P.A. Cammue, Liliane Schoofs, Guoan Luo e reported thatA few plants, including Tujinpi (PseudolarixkaempferiGord.), have extremely strong antifungal activity; each concentrate of this plant can essentially stifle the development of both tried growths. Besides, the CH3)2CO remove from Kushen (Sophora flavescent Ait.), the ethanol, CH3)2CO, and hexane separates from Guanghuoxiang (Pogostemoncablin (Blanco) Benth.) and Gaoliangjiang (AlpiniaofficinarumHance), the hexane extricate from Dingxiang (Eugenia caryophyllataThunb.), and the CH3)2CO, ethanol, and hexane separates from Kulianpi (MeliatoosendanSieb. et Zucc.) and Laliao (Polygonumhydropiper L.) both exhibited a partial development delay of Candida albicans. As our positive control, we used the clinically used antifungal drug miconazole, and in some cases, the growth suppression was even comparable to it. Ends: Most plants whose restorative application as an antifungal treatment is very much upheld.
VI. OBJECTIVES
- To determine safety and efficacy of antifungals.
- To better understand how various antifungal medications work on the molecular level.
- To evaluate the efficacy and safety of these drugs using animal models.
- To provide insights into the development of novel antifungal agents.
VII. RESEARCH ENVISAGED
Resistance and Emerging Fungal Strains: Antifungal resistance is a significant concern, with some fungal species developing resistance to multiple classes of antifungal agents. Research is needed to understand the mechanisms of resistance better and to develop strategies to combat it effectively limited Antifungal Options: There is a relatively limited arsenal of antifungal drugs compared to antibacterial agents. Research is needed to discover and develop new antifungal agents with novel mechanisms of action to expand treatment options.
VIII. HYPOTHESIS
When it comes to treating multidrug-resistant fungal infections, combination therapy with novel synergistic combinations of existing antifungal agents will be more effective than monotherapy. The premise of this hypothesis is that fungal infections that resist standard monotherapy pose a significant clinical problem. By combining antifungal agents with different mechanisms of action, the hypothesis suggests that synergistic effects may lead to enhanced fungicidal activity, overcoming resistance mechanisms and improving treatment outcomes.
The hypothesis also implies that such combination therapies could potentially reduce the risk of developing further resistance in fungal pathogens.
Testing this hypothesis involves experimental studies to evaluate the efficacy, safety, and pharmacokinetics of various combination therapies in relevant animal models and eventually in clinical trials. If proven correct, this research could significantly impact the development of new treatment strategies for managing difficult-to-treat fungal infections.
IX. PLAN OF WORK
Stages of experimental work to obtain the above objectives
- Literature survey
- Study of mechanism of action of various antifungal agents
- Finding of different targets
- Molecular studies
- Animal model studies
- Development of novel antifungal molecule
X. MATERIALS AND METHODOLOGY
A. Materials and Methodology for Antifungal Research
1) Selection of Antifungal Agents
Choose antifungal agents based on their mechanisms of action, spectrum of activity, and relevance to the study objectives (e.g., targeting specific fungal pathogens or resistant strains).
2) Fungal Isolates
Obtain clinical isolates of fungal pathogens relevant to the study (e.g., Candida spp., Aspergillus spp.) or use standard reference strains for consistency.
3) Experimental Models
a) Animal Models in Antifungal Research
Selection of Animal Models
The selection of appropriate animal models is crucial for studying antifungal drugs, as they must mimic the human disease as closely as possible. Different animal models offer unique advantages and challenges.
The blood samples of the three strains of T. asahii (04, 05, and 07) and T. asteroides (01, 06, and 13) used in the study were all isolated. The single T. inkin strain utilized was derived from a white piedra case. Before being tested, the isolates were subcultured twice on Sabouraud dextrose agar (SDA) at 37 degrees Celsius before being stored at 80 degrees Celsius in yeast extract-peptide-dextrose with glycerol. In the case of amphotericin B, the MICs represented a complete inhibition of growth or a significant inhibition of growth (approximately 50% inhibition relative to control growth). In vitro susceptibility testing The in vitro susceptibility of the Trichosporon strains to VRC, PSC, ITZ, FLC, AMB, and CFG (Sigma-Aldrich Brasil Ltd. PZ000. All of the strains were tested using the E-test susceptibility method in accordance with the manufacturer's instructions for VRC, PSC, ITZ, FLC, AMB, and CFG (bioMerieux SA, 532800, 532100, 525808, 510800, 526300, and 532400). Mice and G. mellonella infections were used as experimental infection models. The guidelines that were followed in the development of each and every experiment were established by the Animal Care Committee of the Universiadede Paulo, Campus Preto Paulo, Brazil. The inoculate was made by suspending SDA cultures for 24 hours in sterile saline and filtering them through sterile gauze to get rid of cell clumps or hyphae. The resulting suspensions, which contained 95% of the conidial forms (arthroconidia and blastoconidia), were counted and adjusted to the desired inoculum concentration. Serial dilutions of the initial suspension were cultured on SDA plates to verify the accuracy of the counts.
b) Experimental design
For each strain, 20 groups of 20 larvae were selected at random, and the uninfected control group was challenged with PBS. A Hamilton syringe was used to administer AMB deoxycholate at 0.5 mg/kg of body weight, VRC at 15 mg/kg, and FLC at 20 mg/kg once every four hours after infection.41 Control animals were given 10 ml of PBS. The animals' survival up to 15 days after infection was checked daily. Larvae were examined on a daily basis for 15 days for survival studies. Larvae were cut in half, and 3.7% formaldehyde–PBS was used to fix them for 24 hours. The samples were dehydrated in a series of alcohol solutions before being diaphanized in xylol and embedded in paraffin. Standard procedures were followed to collect 5-mm-thick sections on glass slides and stain them with Gomori methenamine silver (GMS). Deparaffinization, oxidation with 4% chromic acid, methenamine silver solution staining, and light green counterstaining were the primary procedures. Minuscule examinations were performed utilizing an Axioplan 2 imaging magnifying instrument (Carl Zeiss) at the expressed amplifications under splendid field conditions.
- Invertebrates
Galleria mellonella (Greater Wax Moth Larvae): This model is gaining popularity due to its simplicity, ease of use, and ethical advantages. The larvae's immune system shares similarities with the mammalian innate immune response.
- Caenorhabditis elegans
A nematode used for high-throughput screening of antifungal compounds. Its genetic tractability allows for the study of host-pathogen interactions.
- Infection Models
To study the efficacy of antifungal drugs, various infection models are utilized, each replicating different types of fungal infections
- Systemic Infection Models
Candidemia and Invasive Candidiasis: Induced by injecting Candida species into the bloodstream of rodents. This model helps study systemic dissemination and drug efficacy in treating bloodstream infections.
Invasive Aspergillosis: Achieved by inhaling Aspergillus spores, mimicking pulmonary infections common in immune compromised patients.
- Localized Infection Models
Cutaneous and Mucosal Infections: Fungal pathogens such as Candida or dermatophytes are applied topically to the skin or mucosal surfaces. This model is useful for studying superficial fungal infections and the efficacy of topical treatments.
Oropharyngeal Candidiasis: A model for oral thrush, induced by inoculating Candida species in the oral cavity of immunocompromised rodents.
- Biofilm Models
Device-Related Infections: Biofilms on medical devices such as catheters or implants can be studied using animal models. This is crucial for understanding drug efficacy against biofilm-associated infections.
Chronic Wound Models: Used to study the persistence of fungal biofilms in wounds and the effectiveness of antifungal treatments.
- Ethical Considerations
The use of animal models in research necessitates stringent ethical considerations to ensure the welfare of the animals and the validity of the scientific data obtained.
- Replacement, Reduction, and Refinement (3Rs)
Replacement: Using alternative methods such as in vitro models or invertebrates, when possible, to reduce reliance on vertebrate animals.
Reduction: Designing experiments to use the minimum number of animals necessary to achieve statistically significant results.
Refinement: Improving experimental techniques to minimize pain and distress, enhancing animal welfare and data quality.
XI. RESULTS
Validating antifungal research requires various types of data that support the efficacy, safety, mechanisms of action, and clinical relevance of antifungal agents. Here are key types of data commonly used to validate antifungal research:
Evaluation of in vitro antifungal efficacy FLC, ITZ, PSC, VRC, CFG, and AMB's efficacy against the seven Trichosporon isolates was evaluated using the CLSI broth microdilution and the E-test to determine the minimum inhibitory concentration (MIC) (Tables 1, 2, and 3). The MICs determined by the CLSI method and Chagas-Neto et al.26, who examined the same strains of T. asahii and T. asteroides. The MICs for the azoles were different but comparable between the two methods, while the E-test method revealed a lower MIC for AMB and a slightly higher MIC for CFG. The outcomes obtained are consistent with these methods' variations.
In vitro antifungal movement of FLC, ITZ, PSC, VRC, AMB and CFG against all Trichosporon strains utilizing the CLSI stock microdilution strategy.
|
|
Fluconazole MIC(μg/ml) |
Itraconazole MIC(μg/ml) |
||
|
Strain |
24hours |
48hours |
24hours |
48hours |
|
T. asahii o4 |
1.0 |
1.0 |
0.06 |
0.06 |
|
T. asahii 05 |
0.5 |
1.0 |
0.06 |
0.06 |
|
T. asahii 07 |
0.5 |
0.5 |
0.06 |
0.06 |
|
T. asteroides01 |
0.5 |
0.5 |
0.06 |
0.06 |
|
T. asteroides06 |
0.5 |
0.5 |
0.06 |
0.06 |
|
T. asteroides13 |
0.5 |
0.5 |
0.06 |
0.06 |
|
T. inkin |
1.0 |
2.0 |
<0.03 |
0.125 |
Table 1:Trichosporon Strains Movement
|
|
Posaconazole MIC(μg/ml) |
Voriconazole MIC(μg/ml) |
||
|
Strain |
24hours |
48hours |
24hours |
48hours |
|
T. asahii o4 |
0.25 |
0.25 |
<0.03 |
0.06 |
|
T. asahii 05 |
0.125 |
0.125 |
<0.03 |
<0.03 |
|
T. asahii 07 |
0.125 |
0.125 |
<0.03 |
<0.03 |
|
T. asteroides01 |
0.125 |
0.125 |
<0.03 |
<0.03 |
|
T. asteroides06 |
0.125 |
0.25 |
<0.03 |
<0.03 |
|
T. asteroides13 |
0.125 |
0.125 |
<0.03 |
<0.03 |
|
T. inkin |
1.0 |
1.0 |
<0.03 |
0.06 |
Table 2: Trichosporon Strains MovementTop of Form
Bottom of Form
|
|
Amphotericin B MIC(μg/ml) |
Caspofungin MIC(μg/ml) |
||
|
Strain |
24h |
48h |
24h |
48h |
|
T. asahii o4 |
1.0 |
8.0 |
<0.03 |
8.0 |
|
T. asahii 05 |
1.0 |
8.0 |
<0.03 |
16.0 |
|
T. asahii 07 |
1.0 |
1.0 |
<0.03 |
16.0 |
|
T. asteroides01 |
1.0 |
2.0 |
<0.03 |
4.0 |
|
T. asteroides06 |
1.0 |
8.0 |
<0.03 |
16.0 |
|
T. asteroides13 |
1.0 |
4.0 |
<0.03 |
16.0 |
|
T. inkin |
0.5 |
2.0 |
<0.03 |
8.0 |
Table 3: Trichosporon Strains Movement
A. Summary of cell line Studies of above antifungals on Trichosporon Strains
The MIC of amphotericin B was the concentration at which it completely inhibited growth. (100%). For the rest of the drugs, the MIC corresponded to a growth inhibition of 50%.
B. Murine Virulence Model
A murine virulence model was used to evaluate the various Trichosporon species and strains. Again, T. asahii was more virulent than the other two species, which required a larger inoculum or stronger immunosuppression to kill a lot of people. When inoculated at a concentration that was twice that of T. asahii, the three T. asteroides strains displayed a variety of virulence in the murine model. T. asteroides strain 13, one of these, was unable to eradicate the immunosuppressed mice. When using the same inoculum as T. asahii strains, the single T. inkin strain was unable to kill immunosuppressed mice, and when using the same inoculum as T. asteroides strains, it was only able to achieve a 60% mortality rate (6 £ 107 CFU/ml, data not shown). All of the mice died within hours of being infected with a larger inoculum of T. inkin (1 £ 108 CFU/ml), possibly as a result of a physical obstruction of capillaries. A stronger immunosuppression protocol and the same inoculum as T. asahii were used to prevent the animals from dying immediately. This resulted in a mortality rate of approximately 70% and provided a suitable model for evaluating the efficacy of antifungal agents in vivo. Following that, T. asahii strain 07, T. asteroides strain 01, and a single T. inkin strain were chosen to test the efficacy of antifungal agents in vivo.
Histology and VRC decreased the fungal burns in T. asahii- and T. asteroides-infected spleens, respectively. In the case of T. asteroides-infected spleens, VRC was more effective than AMB, while FLC was more effective than VRC in reducing T. asahii and T. asteroides burden in the kidneys. Infected animals' spleens saw more modest reductions in fungus burden. In mice with T. inkin infection, none of the three antifungals significantly reduced the fungal burden in the spleens.
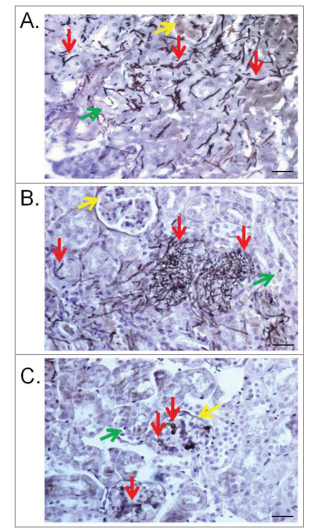
Figure 2: Kidney lesions in the murine model caused by T. asahii 07 (A), T. asteroides 01 (B) or T. inkin (C) infections 6 days after challenge. Transversal sections of the kidney show hyphae, blast conidia, and arthroconidia (red arrows) in the renal tubules (green arrows) and in the glomerular structure (yellow arrows) for some of them. GMS and hematoxylin stain. Bars, 20 mm. Magnification, x400.
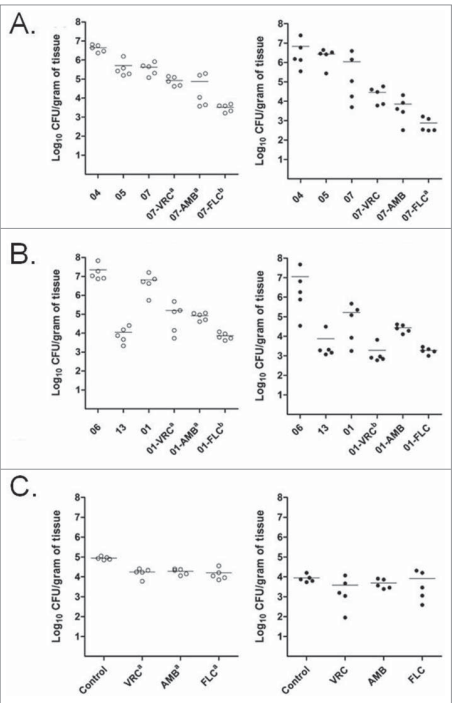
Figure 3: Effects of the antifungal treatments on tissue burden of T. asahii (A), T. asteroides (B) or T. inkin (C) in kidneys and spleen of mice. AMB, deoxycholate at 1.5 mg/kg/day. FLC, at 80 mg/kg/day. VRC, at 60 mg/kg/day. a, P < 0.05 versus control. b, P < 0.05 vs. control and versus one of the other treatments
XII. DISCUSSION
44 reports of the clinical significance of Trichosporon were scarce and inconsistent prior to the genus's taxonomic rearrangement in the 1990s. Trichosporon's species-specific distribution, significance of clinical manifestations, and antifungal susceptibilities were only recently revealed by larger studies that utilized more precise molecular methods for species identification. 26,45 The established Candida breakpoints have been used as a reference to describe the Trichosporon susceptibility results, 28,46,47; however, the clinical and in vitro susceptibility data that are currently available are insufficient to clearly define guidelines for Trichosporon treatments or even susceptibility breakpoints. However, despite the fact that some clinical reports have described the efficacy of CAS against T. inkin when used alone or in combination with AMB, there is a widespread tendency to use azoles, particularly VRC, rather than AMB or the echinocandins2. Over ongoing years a few non-vertebrate creature models have arisen as an option in contrast to the mammalian models of parasitic contamination. These models are a powerful tool for studying fungal pathogenesis, the effectiveness of antifungal compounds, and innate antifungal immunity50. Compared to traditional mammalian models, these non-vertebrate models offer the opportunity to design larger-scale studies at a lower cost and with fewer logistical and ethical constraints. Using genetically modified strains, the present study created a novel G. mellonella infection model for Trichosporon species that can be used to evaluate the efficacy of new antifungal treatments or screen for new virulence factors. The findings from the G. mellonella model can be used in conjunction with those from the murine models. As a result, large-scale studies of non-vertebrates can be used to improve the application of mammalian systems.
A powerful tool for dissecting the molecular virulence mechanisms employed by Trichosporon species and facilitating the identification of novel fungal targets for antifungal therapies to treat this kind of infection will be provided by the recent genome sequencing of T. asahii and the soon-to-be available genome sequences of other Trichosporon species (Goldman et al., forthcoming). As a result, the current study's combination of G. mellonella and murine assays will be useful for studying Trichosporon virulence and improving antifungal strategies for controlling trichosporonosis in the future..
Conclusion
Antifungal research is a dynamic and critical field, addressing the growing challenge of fungal infections, which range from superficial to life-threatening systemic diseases. Over the years, significant advancements have been made in understanding and developing antifungal drugs, but several challenges remain. Antifungal research is a dynamic and critical field, addressing the growing challenge of fungal infections, which range from superficial to life-threatening systemic diseases. Over the years, significant advancements have been made in understanding and developing antifungal drugs, but several challenges remain. In the murine model, the lower virulence of T. asteroides and T. inkin was correlated with the delayed killing rate of G. mellonella larvae compared to T. asahii. The fact that two of the three strains of T. asteroides failed to produce a high mortality rate, in contrast to the high fungal burden in kidneys that was comparable to that of T. asahii, was a remarkable observation. Clinical data from a number of studies indicate that T. asahii is more likely than T. asteroides or T. inkin to cause deep-seated infections and more fatal infections in the case of disseminated infections. It is interesting to note that T. asteroides strains 06 and 13 are less virulent than strain 01. T. asteroides strains 06 and 13 have recently been shown to produce less biofilm than T. asteroides strain 01; these differences may be related to the differences in virulence between these strains. These strains\' genomes are currently being sequenced, and functional studies can help us understand why they have different virulence capabilities by comparing them. All strains were susceptible to azole drugs, and similar results were obtained using both the E-test and the microdilution methods. The antifungal susceptibility results obtained in this study were within the range previously described for these Trichosporon species. In the murine model, where both FLC and VRC demonstrated some degree of efficacy in treating the disseminated Trichosporon infection, the in vitro antifungal sensitivity results are correlated with the mortality reduction. Despite high doses, none of the tested antifungals completely eradicated the infection. VRC had lower MICs than FLC, but it did not improve mice\'s survival against two of the three Trichosporon species as well. This may be due to the inherent difficulties of testing VRC in the murine model, where VRC levels in the serum rapidly degrade even when grapefruit juice is given to the animals, which has been shown to increase serum levels of this drug up to therapeutic levels. In contrast, VRC performed better against T. asahii infections in an alternative mammalian model, the guinea pig, even at a lower dose. In contrast, G. mellonella survival infected with all three Trichosporon species was enhanced by both VRC and FLC. AMB\'s overall performance in the murine model was comparable to that of VRC and slightly lower than FLC, but its MICs against all of the stains tested were higher than those of the azoles in the current study.
References
[1] Nazzaro F, Fratianni F, Coppola R, De Feo V. Essential oils and antifungal activity. Pharmaceuticals. 2017 Nov 2;10(4):86. [2] Zhao Z, Wang Q, Wang K, Brian K, Liu C, Gu Y. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresource technology. 2010 Jan 1;101(1):292-7. [3] Sortino M, Delgado P, Juárez S, Quiroga J, Abonía R, Insuasty B, Nogueras M, Rodero L, Garabito FM, Enriz RD, Zecchino SA. Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorganic & Medicinal Chemistry. 2007 Jan 1;15(1):484-94. [4] Kurita N, Miyaji M, Kurane R, Takahara Y. Antifungal activity of components of essential oils. Agricultural and Biological Chemistry. 1981;45(4):945-52. [5] Quiroga EN, Sampietro AR, Attune MA. Screening antifungal activities of selected medicinal plants. Journal of ethnopharmacology. 2001 Jan 1;74(1):89-96. [6] Liu Q, Luyten W, Pellens K, Wang Y, Wang W, Thevissen K, Liang Q, Cammue BP, Schoofs L, Luo G. Antifungal activity in plants from Chinese traditional and folk medicine. Journal of ethnopharmacology. 2012 Oct 11;143(3):772-8. [7] Hartsel, S., & Bollard, J. (1996). Amphotericin B: new life for an old drug. Trends in Pharmacological Sciences, 17(12), 445-449. [8] Ellis, D. (2002). Amphotericin B: spectrum and resistance. Journal of Antimicrobial Chemotherapy, 49(Supple 1), 7-10. [9] Perfect, J. R., & Bicanic, T. (2015). Cryptococcosis diagnosis and treatment: what do we know now.Fungal Genetics and Biology, 78, 49-54. [10] Pfaller, M. A., & Diekema, D. J. (2007). Epidemiology of invasive candidiasis: a persistent public health problem. Clinical Microbiology Reviews, 20(1), 133-163. • Wiederhold, N. P. (2017). Antifungal resistance: current trends and future strategies to combat. Infection and Drug Resistance, 10, 249-259. [11] Perfect, J. R. (2017). The antifungal pipeline: a reality check. Nature Reviews Drug Discovery, 16(9), 603-616. [12] Lionskins, M. S., & Levitz, S. M. (2017). Host control of fungal infections: lessons from basic studies and human cohorts. Annual Review of Immunology, 35, 157-191. [13] Patterson, T. F., Thompson, G. R., Denning, D. W., Fishman, J. A., Hadley, S., Herbrecht, R., ...& Walsh, T. J. (2016). Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clinical Infectious Diseases, 63(4), e1-e60. [14] Pappas, P. G., Kauffman, C. A., Andes, D. R., Clancy, C. J., Marr, K. A., Ostrosky-Zeichner, L., ... & Sobel, J. D. (2016). Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clinical Infectious Diseases, 62(4), e1-e50.
Copyright
Copyright © 2024 Pooja Kumari, Sujata Kushwaha, K. C. Tyagi, Narendra Patel. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64031
Publish Date : 2024-08-21
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

